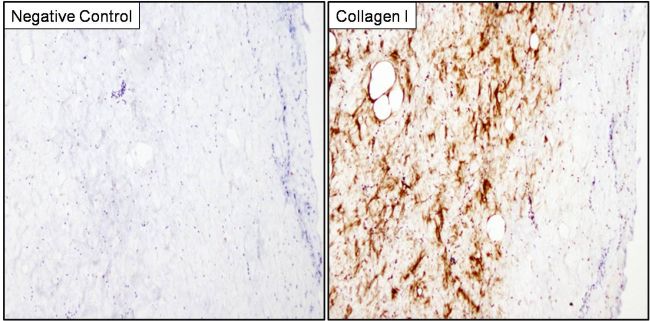
Immunohistochemistry analysis of Collagen I was performed on porcine skin tissue. To expose target proteins, antigen retrieval was performed by microwaving tissues for 8-15 minutes in 10mM sodium citrate buffer (pH 6.0). Following antigen retrieval, tissues were blocked in 3% hydrogen peroxide-methanol for 15 min at room temperature, washed with deionized water and PBS, and then probed with a Collagen I monoclonal antibody (Product # MA1-141) diluted 1:20 in 3% BSA-PBS (right panel) or incubated with buffer alone not containing primary antibody as a negative control (left panel), overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
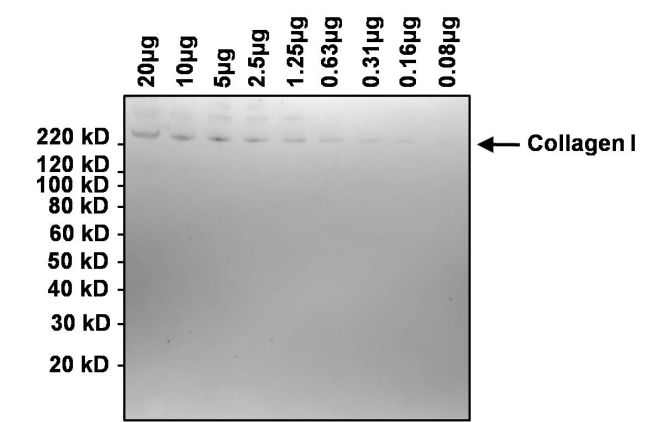
Western blot analysis of collagen I was performed by loading the indicated amounts of bovine collagen I onto a 4-12% Bis-Tris polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane and blocked with 5% BSA in TBST for at least 1 hour. The membrane was probed with a collagen I monoclonal antibody (Product # MA1-141) at a dilution of 1:1000 overnight at 4°C on a rocking platform, washed in TBST, and probed with an HRP-conjugated goat anti-mouse IgG secondary antibody (Product # 31430) at a dilution of 1:20,000 for 1 hour. Chemiluminescent detection was performed using SuperSignal West Dura (Product # 34076).
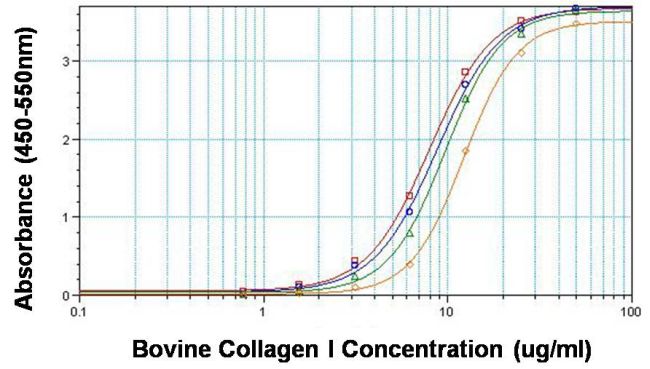
Direct ELISA analysis of collagen I was performed by coating 100ul per well of bovine collagen I in twelve replicates at 50, 25, 12.5, 6.25, 3.12, 1.56, 0.781 and 0ug/ml across a clear, 96-well plate and incubating overnight at room temperature. The coating solution was decanted and the plate was blocked with 5% BSA in TBST for at least 2 hours. The plate was washed with TBST and then incubated with 100ul per well of collagen I monoclonal antibody (Product # MA1-141) at 50ug/ml (red line), 10ug/ml (blue line), 2ug/ml (green line) and 0.4ug/ml (orange line) in blocking buffer for 1 hour at room temperature. The plate was washed and incubated with 100ul per well of HRP-conjugated goat anti-mouse IgG secondary antibody (Product # 31430) in all test wells at 1:10,000 for 30 minutes at room temperature. The plate was again washed in TBST and detection was performed using ultra TMB substrate (Product # 34028) for 30 minutes at room temperature in the dark. The reaction was then stopped with

Immunohistochemistry analysis of Collagen I was performed on frozen human breast carcinoma tissue. Tissues were probed with a Collagen I monoclonal antibody (Product # MA1-141, right panel) or mouse IgG isotype control (left panel) at a dilution of 1:500 for 1 hour at room temperature. Tissues were washed, and detection was performed using an HRP-conjugated universal detection reagent followed by DAB substrate. Tissues were counterstained and prepped for mounting before visualization by light microscopy.



